The Food and Drug Administration (FDA) has allowed marketing for the first-ever government-approved mobile medical app designed to help people with substance use disorders (SUD).
The San Francisco- and Boston-based app developer Pear Therapeutics created reSET, a digital app that focuses on outpatient therapy to aid against relapse from cocaine, stimulants, alcohol and marijuana use. However, the app is not yet designed to treat opioid addiction.
“It’s a prescription therapy so it will be available via prescription, and [patients] participate in a 90-day treatment course,” said Corey McCann, CEO of Pear Therapeutics.
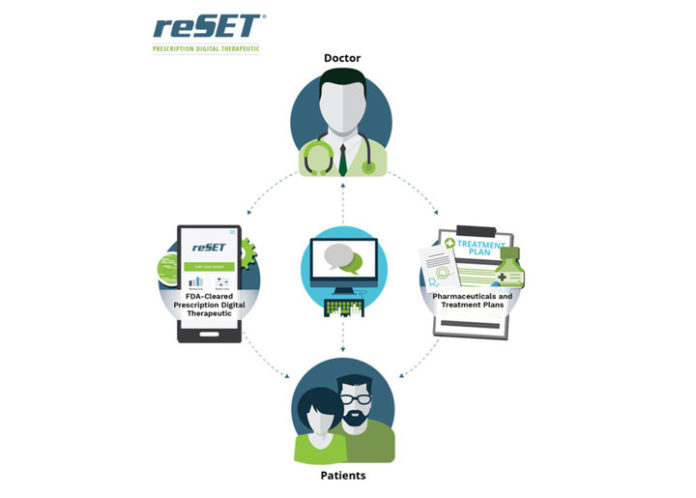
The reSET app contains a patient application and a clinician dashboard. It provides cognitive behavioral therapy that teaches important skills used to continue treatment of a SUD, improve abstinence from drug use, and boost retention in outpatient programs.
The FDA analyzed data from a 12-week trial with nearly 400 patients who participated in standard addiction treatment or standard treatment alongside a desktop version of reSET. The results revealed a noticeable increase in sustained abstinence for those who used the app — 40.3 percent versus 17.6 percent, the government agency stated in a press release.
“I think if you roughly look at the size of the problem, we’re treating about 10 percent of the people that need treatment for SUD,” he said. “It’s a critical time for us to scale these types of products to bring [reSET] to all types of people.”
The federal agency emphasized the application’s use in conjunction with outpatient therapy and a contingency management system, which is a commonly used method to treat addiction that promotes adherence through a reward system.
The FDA noted that reSET does not currently assist people with only an alcohol dependence or those with a primary addiction to opioids.
“The clinical outcomes demonstrated in the reSET pivotal study are remarkable,” stated Dr. Edward Nunes, lead investigator on reSET’s clinical study, in a press release by Pear Therapeutics. “Clinically-validated digital therapeutics may become… (continue reading)